| Country/Area | By |
|---|---|
| USA | FDA 510(K) |
| European Union | CE mark |
| Canada | Health Canada |
| Australia | TGA |
| New Zealand | WAND |
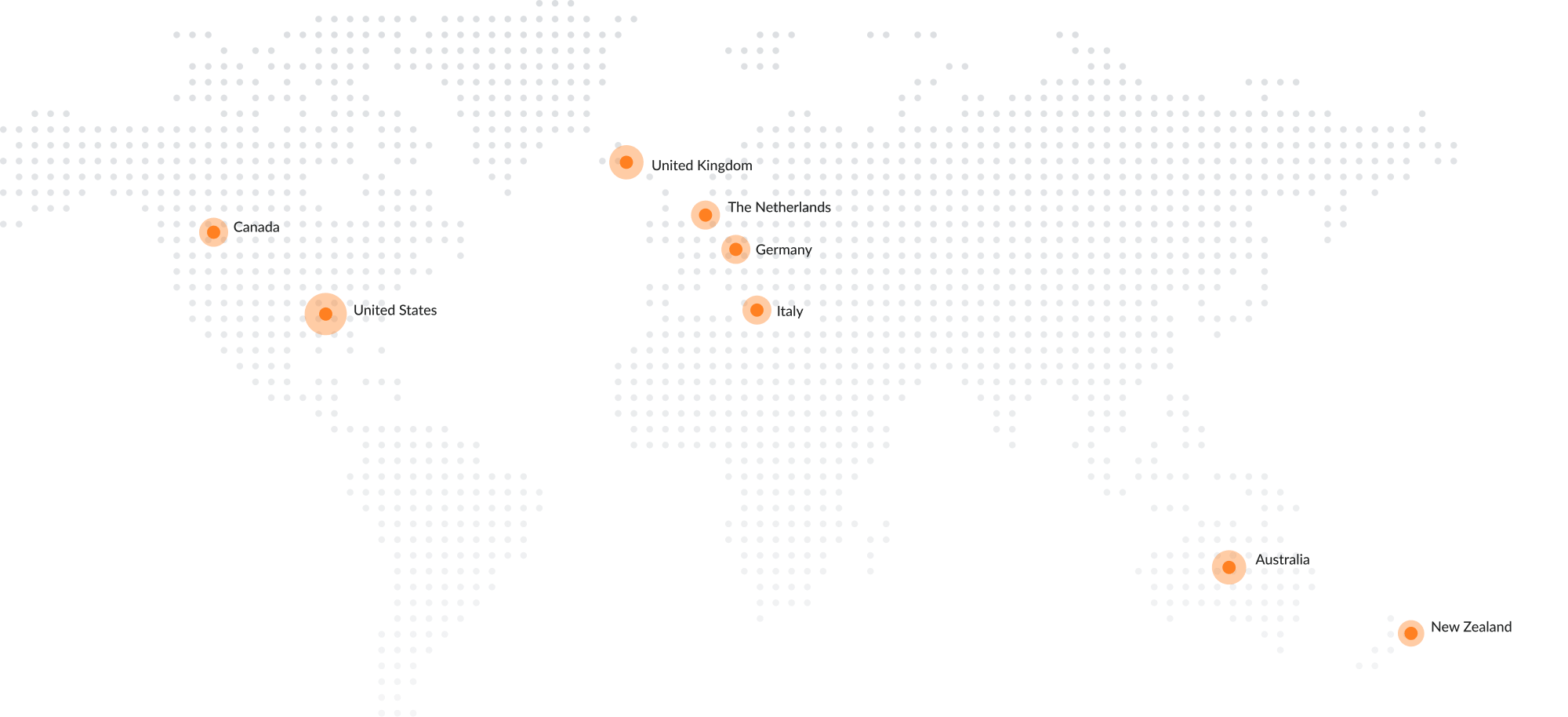
A personal approach with global reach
The world leading Dario Blood Glucose Monitoring System has US FDA clearance and approval
from the European CE, Health Canada, and Australia’s TGA.
Dario products are approved to sell in the United States, Canada, the United Kingdom, Germany, Italy, Australia, The Netherlands, and New Zealand. DarioHealth is rapidly moving into new geographic markets and chronic conditions including hypertension, stress management and obesity.